A molecular camera allows real-time imaging of cancer resection margins
Currently, over three million people are diagnosed with cancer each year across Europe. Healthcare costs exceed EUR 125 billion, posing a significant economic challenge. Despite medical advances, the primary mode of treatment for most cancer types is surgery.
Imaging that enlighten surgeons
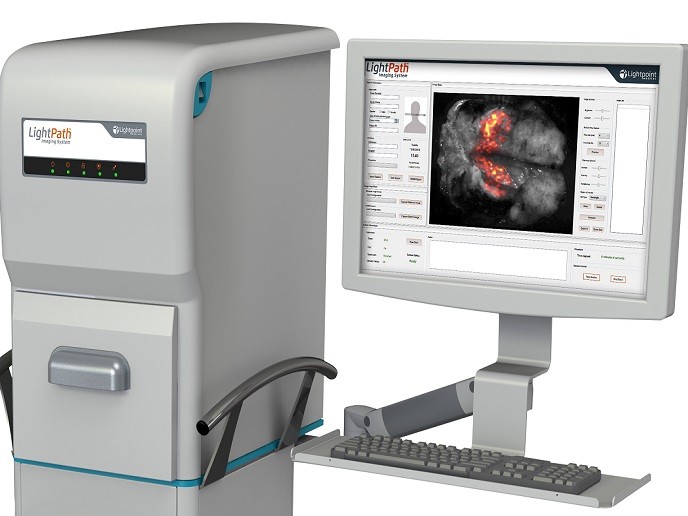
The EU-funded CerISMA(opens in new window) project tested the clinical efficacy of an innovative technology that assists with the surgical resection of cancer in a single operation. The LightPath™ imaging sytem developed by Lightpoint Medical Ltd in the UK can detect the uptake of positron-emission tomography (PET) imaging agents commonly used in pre-operative scans. As project coordinator Joanne O’Shea explains, “LightPath™ enables surgeons to differentiate cancer from normal tissue and define cancer margins during surgery.″ The LightPath™ molecular imaging camera can detect Cerenkov light, a phenomenon first described in the 1930s. Cerenkov light appears when a charged particle travels through matter such as water or tissue faster than light. However, it wasn’t until 2009 that scientists discovered that PET imaging agents – used nowadays for cancer diagnosis – produced the same visible light.
Imaging agents define surgery margins
Prior to surgery, the patient is administered the imaging agent, which selectively accumulates in cancerous cells. LightPath™ rapidly images the tumour specimen immediately after excision. As imaging agents travel only a few millimetres in biological tissue, the LightPath™ system depicts positive signals from cancerous cells only within a few millimetres of the tissue's surface, providing clear resection margins. In particular in breast cancer, complete removal of the tumour is critical but histological examination of the resected tissue can take up to two weeks. Positive margins are detected in nearly a quarter of the patients who are advised to undergo re-operation and/or receive further adjuvant therapies. To assess the efficacy of LightPath™, the CerISMA project undertook a clinical study.
Lighting the path to the future
The LightPath™ imaging system has been tested in a pilot breast cancer trial at Guy’s & St Thomas’ Hospital in London and in a larger-scale study with over 60 patients at 3 hospitals across Poland. Results demonstrated that it can be safely and effectively used for surgical margin assessment in breast surgery. In general, LightPath™ provided good quality images while staff radiation exposure was low and within acceptable limits. Overall, CerISMA produced invaluable results that will be instrumental to the further development of LightPath™ technology. During the project, the company secured ‘early adopter’ sales of the imaging system in Germany, the Netherlands and United Kingdom. Ongoing result evaluation in conjunction with emerging data from current pilot clinical trials in prostate cancer and head and neck cancer surgery will further help shape the device commercialisation strategy. In view of the future, O’Shea is confident that “the LightPath™ imaging system will offer a dramatic reduction in costly re-operations and adjuvant therapies across a wide range of major cancer types.″ The device will not only comprise an innovative solution for healthcare providers, but will also substantially reduce cancer care costs.