Mechanistic insight into fat regulation: implications for chronic liver diseases
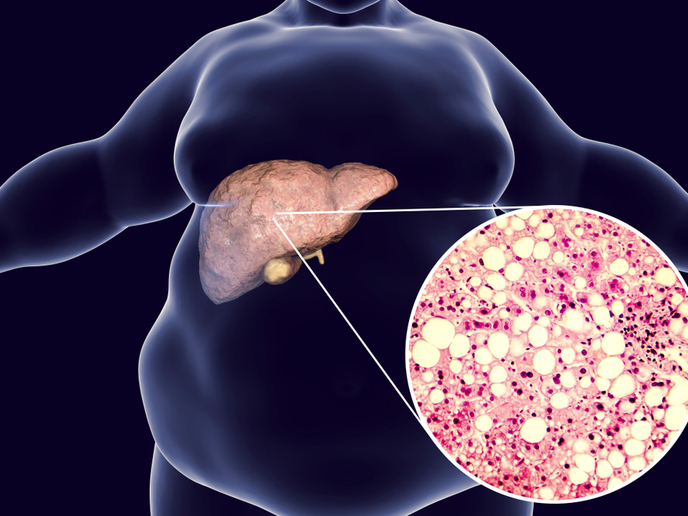
The liver is the central organ that controls lipid homeostasis and metabolises fatty acids into triglycerides(opens in new window) (TGs). Hepatocytes package TGs in small quantities in organelles known as lipid droplets (LDs), and when required to maintain lipid levels and meet metabolic demands, the body mobilises TGs from LDs. The capacity to store TGs and maintain an internal steady state of lipids is key for survival when nutrient supply is limited. However, increased energy intake combined with decreased energy expenditure leads to several common metabolic disorders, including obesity and type 2 diabetes. In turn, these can cause non-alcoholic fatty liver disease(opens in new window) (NAFLD), a chronic condition associated with high accumulation of hepatic TGs. Given the serious NAFLD complications, which include non-alcoholic steatohepatitis(opens in new window) (NASH) and cirrhosis, it is of fundamental importance to study the mechanisms that promote hepatic lipid accumulation.
Insight into key enzymes of lipid biosynthesis
Scientists of the TGDNL project were interested to understand how cells regulate and coordinate lipid storage to achieve energy homeostasis. The research was undertaken with the support of the Marie Skłodowska-Curie Actions programme and focused on the crosstalk between lipid storage and biosynthesis. “TGDNL findings have the potential to provide new therapeutic strategies for diseases characterised by perturbed lipid storage,” explains research fellow Mikael Rydén. The TGDNL project was a collaborative effort with Robert Farese and Tobias Walther(opens in new window) at the Harvard T.H. Chan School of Public Health. The scientific teams used both cellular and animal models to investigate the role of diglyceride acyltransferase(opens in new window) enzymes (DGATs) which catalyse the final and only committed step in TG synthesis. Advanced molecular techniques alongside pharmacological inhibition or genetic loss-of-function of DGATs showed that reduced TG storage resulted in attenuated fatty acid synthesis. This feedback loop seems to be regulated by sterol regulatory element-binding proteins(opens in new window) (SREBPs), transcription factors that regulate the expression of genes involved in lipid synthesis. Inhibition of DGAT2 directly affected the levels and activity of SREBP alongside genes implicated in lipid biosynthesis.
TGDNL clinical impact and future directions
“Our findings highlight a universal feedback mechanism wherein cellular lipid storage controls fatty acid synthesis, providing important insight into the regulatory mechanisms of cellular lipid homeostasis,” emphasises Rydén. These findings may be of relevance for the future design and development of effective pharmacological treatments against NAFLD. This is of great importance since there are currently no effective therapies for NAFLD apart from lifestyle changes that can improve physical fitness and weight loss. Nonetheless, the pathophysiological relevance of the mechanisms deciphered during TGDNL, and especially the role of DGATs and SREBPs in common metabolic disorders, warrants further investigation. To achieve this, scientists plan to analyse multiple omics data and associate them with functional phenotypes of adipose tissue and liver obese patients with or without type 2 diabetes or metabolic syndrome.